An Overview of Peptides, Their Applications, and Their Purpose
Peptides are molecules that consist of between 2 and 50 amino acids. They may be subdivided into oligopeptides, which have few amino acids (e.g., 2 to 20), and polypeptides, which have many amino acids.
There are two main differences between peptides and proteins. The first being that peptides are made up of smaller chains of amino acids. Proteins can either be one long chain of 100 or more amino acids, or they can be made of several amino acid chains joined together. Amino acids that have been incorporated into peptides are termed “residues.” All peptides have an N-terminal and C-terminal residue at the end of the peptide. The type and sequence of amino acids that compose the chain will then determine the function that the peptide performs.
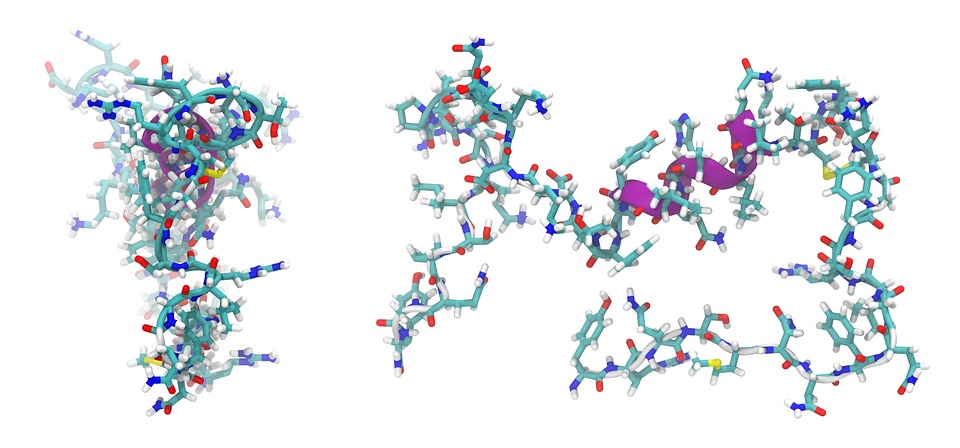
The second dissimilarity between peptides and proteins is that proteins can adopt more complex formations known as secondary, tertiary, and quaternary structures. However, peptides are generally linear and less defined.
Peptides can be categorized according to their sources and function. These molecules carry out crucial biological functions including DNA replication, transporting molecules, catalyzing metabolic reactions, among others. They are involved in many metabolic pathways and are the main components for a vast number of reactions that occur within our body.
Peptides in Their Natural State
Peptides are found in all living organisms and are synthesized from the transcription of a sequence in our genetic code. Some of their most relevant functions are:
- Precursors of proteins
- Constituents of alkaloids
- Hormones
- Antioxidants
- Growth factors
Normal concentrations of peptides contribute to the stabilization of metabolic pathways and to balance the reactions that occur within the body. Cellular events such as gene expression, protein synthesis, degradation, and DNA proofreading are also regulated through peptide or protein interactions. Peptides, hormones, and neurotransmitters behave as the first messengers that trigger cell signal transduction that subsequently affects the cellular response and expression of effector genes.
The Contribution of Peptides in Research Advances
The analysis of the molecular interaction of peptides has always been considered crucial for successful drug synthesis. Development of sophisticated equipment has contributed to the understanding of binding interactions and the consequent improvement of peptide drugs, which function by interacting with a vast number of targets such as proteins, nucleotides, lipids or metabolites.
Peptide drugs generally work as inhibitors against protein-protein interaction and can derive from the binding region of an inhibitor protein. Science’s efforts are oriented towards enhancing the specificity and selectivity of these molecules. The low toxicity of peptides has even further helped increase their popularity. Today, there are more than 50 peptide drugs that have been approved for clinical use.
Some of the most popular peptide drugs are glatiramer acetate (employed to treat multiple atherosclerosis), leuprolide acetate and goserelin acetate (used in the treatment of different types of cancers), and exenatide (administered to patients with diabetes mellitus).
Cyclotides are a variant of peptides that have also gained prestige in the scientific world. They are also comprised of a string of amino acids, but the main difference with traditional peptides lies in the fact that the ends are joined together to form a circle. The relevant aspect of cyclotides is that they do not have any loose ends, the weak point that speeds up degradation by our enzymes.
These findings are just a few reasons to believe that what has been explored so far about peptide drugs is just the beginning. There will certainly be more generations of drug development to come that will enable us to treat more complex diseases using peptides.
SOURCES: